RESEARCH ARTICLE | https://doi.org/10.5005/jp-journals-10025-1174 |
Analysis of the Change Induced by Riboflavin and Ultraviolet Light on Corneal Collagen by Infrared Spectrometry
1–3,5Department of Ophthalmology, University of Saskatchewan, Saskatoon, Saskatchewan, Canada
4Department of Geological Sciences, University of Saskatchewan, Saskatoon, Saskatchewan, Canada
Corresponding Author: Vinay Kansal, Department of Ophthalmology, University of Saskatchewan, Saskatoon, Saskatchewan, Canada, Phone: +1 306 261 4226, e-mail: vkans061@uottawa.ca
How to cite this article Kansal V, Lukenchuk J, Dodd M-MU, et al. Analysis of the Change Induced by Riboflavin and Ultraviolet Light on Corneal Collagen by Infrared Spectrometry. Int J Kerat Ect Cor Dis 2019;8(1):17–22.
Source of support: College of Medicine, University of Saskatchewan
Conflict of interest: None
ABSTRACT
Aim: Corneal collagen cross-linking (CCL) is a procedure that exposes the cornea to ultraviolet light and/or riboflavin to halt the progression of corneal ectatic disease. Currently, most investigations using Fourier-transform infrared spectroscopy (FTIR) of corneal changes following CCL focus on corneal ultrastructure, and not on changes at the molecular level. The aim of this study was to investigate the temporal and spatial separation of corneal collagen linkages that underlie the success of CCL.
Materials and methods: Controlled experimental trial. Pairs of donor globes from five patients (n = 10) were divided into interventional and control groups. Interventional group corneas (n = 5) were exposed to riboflavin 0.1% and ultraviolet-A (UVA) light according to the modified Dresden protocol, harvested, cryo-microtomed, and placed on glass slides. Control group corneas (n = 5) underwent cryo-microtoming without CCL. Molecular changes were imaged using the synchrotron mid-infrared beamline at the Canadian Light Source.
Results: Fourier-transform infrared spectroscopy imaging of total protein, integrated area under the amide I band from 1,700 to 1,600 cm−1, FTIR imaging of collagen triple helix structures, second-derivative intensity as 1,666 cm−1, and FTIR imaging of aggregated proteins, second-derivative intensity as 1,625 cm−1 detected no difference in intramolecular cross-links between the interventional and control corneas. The secondary structure of collagen was neither significantly altered nor was its evidence of aggregation or denaturation within the cornea.
Conclusion: Our data suggest that intramolecular cross-linking does not play a major role in CCL and that it is more likely to increase in intermolecular linkages that accounts for increased corneal strength.
Clinical significance: An increase in intermolecular linkages likely accounts for the increased corneal strength observed following CCL. We hope that these results will guide future work to optimize techniques for CCL.
Keywords: Corneal collagen, Corneal collagen cross-linking, Keratoconus, Riboflavin, Ultraviolet light.
INTRODUCTION
Keratoconus is a non-inflammatory, progressive, ectatic disease of the cornea resulting in irregular astigmatism and myopia, affecting 1 in 2,000 Canadians.1 In the normal cornea, collagen cross-linking (CCL) is an anatomical function of collagen fibrils to establish strong adherence to neighboring fibrils. In keratoconus, the structural integrity of the corneal collagen scaffold is weakened, resulting in the focally reduced radius of curvature, abnormal wavefront aberrations, and a reduction in corneal stiffness and thickness. Ultimately, aberrations can result in significant reductions in best-corrected visual acuity, which may eventually require corneal transplantation.2–5
Corneal CCL is a procedure whereby the photochemical reaction between ultraviolet-A (UVA) light and a photosensitizer, riboflavin, is used to increase the biomechanical rigidity of the cornea. First introduced by Spörl et al.,6 this is only the treatment approach capable of significantly altering disease progression and preventing the need for eventual corneal transplantation.5,6 The current standard approach to CCL is the Dresden protocol, whereby the cornea is treated with 3 mW/cm2 UV intensity over 30 minutes.7 Other accelerated protocols have been developed with purportedly equivalent efficacy.8,9
Although CCL is an approved therapeutic method in Canada, USA, and Europe, and the chemical process of cross-linking is well understood, the exact spatial and temporal mechanism of CCL in situ, following treatment with riboflavin and ultraviolet light, is incompletely understood. Previous studies report treatments of riboflavin and UVA light can alter the molecular scaffolding in the cornea; investigators hypothesize that both intermolecular and intramolecular linkages of collagen fibrils are responsible for increased corneal strength posttreatment.10–15 The synchrotron mid-infrared beamline using Fourier-transform infrared spectroscopy (FTIR) imaging offers the opportunity for direct molecular imaging in situ at the cellular spatial resolution the changes induced by CCL. Previous laboratory-based investigations have focused on the effects of CCL on the corneal ultrastructure; there is a lack of understanding of the specific molecular changes induced by CCL.3,7,10,11,16,17 The main purpose of this study is to investigate the temporal and spatial separation of the collagen linkages in the cornea that underlie the success of this therapy. We further sought to investigate the ultimate depth at which riboflavin and ultraviolet light treatment influence the rigidity of the corneal structure.
MATERIALS AND METHODS
Human Cornea Collection, Dissection, and Accelerated Cross-linking Procedure
With approval from the Biomedical Research Ethics Board at the University of Saskatchewan, 10 donor globes were provided by the Lions Eye Bank of Saskatchewan from five different donors. Pairs from each donor were split up into control and intervention groups.
The intervention group underwent an accelerated corneal cross-linking procedure (modified Dresden protocol).18 The epithelium was removed with spears. VibeX Rapid (Simovision BVBA, Overijse, Belgium), formulation of riboflavin (0.1%) was applied until the exposed stroma was completely covered, followed by applications of one drop every 2 minutes for 10 minutes total. Corneal thickness was measured, and the globes were rinsed with a balanced salt solution. UVA treatment was applied using the KXL system (Simovision BVBA, Overijse, Belgium). Four minutes of irradiation at 30 mW/cm2 was applied for a total treatment dose of 7.2 J. The cornea was moistened throughout the irradiation procedure with balanced salt solution (BSS) as required. Corneas were then excised from the globes.
Sample Preparation
To avoid the introduction of chemical artifacts that can result in sample preparation of biological samples, all cornea tissue immediately flash frozen through immersion in liquid nitrogen-cooled iso-pentane. 10 μm thick sections of the cornea were cut with a cryo-microtome at −18°C; the sections were melted onto the substrate. Two adjacent serial sections were cut for each sample, one melted onto a CaF2 substrate for FTIR analysis, the other melted on a regular glass microscope slide for routine hematoxylin and eosin (HandE) histology analysis. The tissue sections were air-dried in an ambient laboratory environment at ambient room temperature (~23.5°C) and analysed using FTIR immediately or stained with HandE immediately (within 24 hours of tissue sectioning).
Validation of UV Treatment Efficacy
In order to ensure that corneas were treated during the accelerated cross-linking procedure, a separate experiment of the trypsin digestion of collagen was performed. Tissue sections were cut at 10 and 30 μm and treated with trypsin or a blank buffer for either 2, 10, and 30 minutes. Samples were then rinsed in buffer, fixed with 4% buffered formalin and stained with HandE. The intensity of HandE-stained was used as a relative indicator of protein content, that is, the more pink the tissue the more protein content. The aim was to demonstrate that UV-treated samples would appear “more pink” after trypsin treatment compared to the untreated samples.
Corneal sections for FTIR imaging were mounted on glass microscope slides and were postfixed in 4% buffered formalin and routine HandE histology was performed.
Large Area Globar-FTIRI
The moderate spatial resolution of large areas of corneal tissue was collected images were collected from five cornea tissue sections in the untreated group and five cornea tissue sections in the treated group. The images were collected using a focal plane array (FPA) detector and globar source. The globar-FTIR-FPA spectroscopic images were collected at the Canadian Light Source (CLS) using a Hyperion 3000 microscope fitted with an upper objective of 15× magnification and a numerical aperture of 0.6, and a lower condenser of 15× magnification and 0.4 numerical aperture. This arrangement yielded a pixel size of 2.65 μm, which was later subjected to 8 × 8 pixel binning to yield an effective image pixel size of 21.4 μm. The FTIRI data were collected with a spectral resolution of 4 cm−1 and the coaddition of 32 scans, and a background image was collected from a blank substrate using 32 coadded scans. The background was collected immediately before each sample.
High-resolution Single-beam Synchrotron Radiation-FTIRI
High spatial resolution SR-FTIR images were collected from five cornea tissue sections in the untreated group and five cornea tissue sections in the treated group. Single-beam wide-field SR-FTIRI was performed at the CLS using a Hyperion 3000 microscope fitted with an upper objective of 52× magnification and a numerical aperture (NA) of 0.65, and with a lower condenser of 32× magnification and an NA of 0.65. This arrangement yielded 0.77 μm × 0.77 μm pixel size. The infrared beam was focused and aligned to the center of the array, and then defocused such that an array area of approximately 25 μm × 25 μm (32 × 32 pixels) was quasi-homogeneously illuminated. To image larger sample regions, this infrared beam was “stepped” across the sample to generate an image mosaic.
Spectra were collected with the coaddition of 256 scans at 4 cm−1 resolution. A background spectrum (256 coadded scans) was collected from the blank substrate before each sample measurement to minimize the effect of the beam’s current decay on data.
Data Processing and Data Analysis of FTIR Spectra
All data processing and image generation were performed using Cytospec software (Cytospec, Version 1.2.04) and Opus software (Version 6.5, Bruker, Ettlingen, Germany). False-color functional group images of total protein were generated from the integrated band area in non-normalized spectra. Areas were integrated with the range 1,700–1,600 cm−1 for the protein amide I band. A linear baseline was subtracted across the same range as integration. To calculate the average spectrum a region of interest was drawn to encompass the region of interest. Drawing the region of interest was guided by an overlay of HandE histology and FTIR images of total protein.
RESULTS
Validation of Cross-linking Efficacy—Trypsin Test
The validation protocol demonstrated that collagen content increased with increased tissue thickness, as evidenced by more intense eosin staining. Increased trypsin incubation time decreased corneal collagen content. Both results taken together support the efficacy of the test itself. The CCL-treated corneas stained more intensely than the control corneas, demonstrating that the collagen in the treated corneas is more resistant to digestion (Fig. 1).
FTIR Imaging of Total Protein in the Cornea Compared with Sclera
Fourier-transform infrared spectroscopy imaging demonstrated increased protein in the sclera relative to the cornea, in images collected at spatial resolutions of 40, 20, 5, and 0.77 μm. Images at 0.77 μm represent the theoretical maximum resolution of the synchrotron mid-IR beam. Bright-field imaging of HandE-stained tissues demonstrated the opacity of the scleral tissues (appearing dark under light-microscope) due to the irregular configuration of collagen fibrils. Imaging of corneal tissue demonstrated the parallel collagen fibril alignment (Fig. 2).

Figs 1A to C: HandE histological evaluation of relative collagen content and the effect of UV treatment on collagen stability: (A) Collagen content increases with tissue thickness and results in a more intense eosin staining; (B) Increased trypsin incubation time decreases cornea collagen content and reduces eosin intensity; (C) UV and riboflavin crosslinking of collagen in cornea tissue minimizes trypsin digestion of collagen and leads to a more intense eosin stain, relative to untreated tissue
FTIR Imaging of Total Protein, Collagen Triple Helix and Aggregated Protein in the Cornea
Fourier-transform infrared spectroscopy imaging detected no difference between the treated and untreated corneas with respect to (1) of total protein, integrated area under the amide I band from 1,700 to 1,600 cm−1, (2) collagen triple helix structures, second-derivative intensity as 1,666 m−1, (3) aggregated proteins, second-derivative intensity as 1,625 cm−1 (Fig. 3).
FTIR Spectra of Secondary Protein Structures
The composition of secondary protein structures in treated and untreated tissues was further elucidated by quantifying the FTIR spectra of each secondary structure of interest, and curve fitting the amide I band to the data. The analysis revealed nearly identical secondary protein structures between the treated and untreated corneas. Verification against the untreated scleral sample revealed different secondary structures of the collagen proteins (Fig. 4). These results also suggest that the CCL procedure does not appear to be inducing any protein aggregation.

Figs 2A to E: FTIR imaging of total protein in cornea tissue section; (A) Bright field image of HandE-stained cornea tissue section. More intense eosin staining is observed in the sclera indicating a higher protein content. Inset shows that the sclera appears dark and is not transparent in the bright field image of the unstained tissue, while the cornea itself is transparent; (B to E) FTIR images of total protein, which show increased protein in the sclera relative to the cornea. The FTIR images were collected at different spatial resolution: (B) 40 μm; (C) 20 μm; (D) 5 μm; and (E) 0.77 μm. Images (B) to (D) were collected using a nonsynchrotron thermal infrared source, image (E) was collected with a synchrotron infrared beam, at the Canadian Light Source
DISCUSSION
In brief, FTIR imaging was not able to detect any differences in the collagen structure between untreated and treated corneas. We used trypsin digestion of collagen and HandE histology to confirm that the UV treatment had worked, that is, the collagen in UV-treated corneas was more resistant to trypsin digestion. These results taken together with the FTIR data suggest that CCL improves corneal strength by causing the formation of crosslinks between collagen fibrils (intermolecular bonds) rather than within the same collagen fibril (intramolecular bonds).
This contrasts with previous investigators who hypothesized that both intermolecular and intramolecular linkages of collagen fibrils are responsible for increased corneal strength post-CCL. Specifically, previous reports have suggested that the formation of dityrosine, a molecule capable of forming intermolecular and intramolecular linkages between collagen fibrils, is formed in CCL.10–15 However, prior to the present study, investigators have not shown which types of bonds are specifically formed. Furthermore, data from Wollensak et al. demonstrate that CCL does not increase interlamellar cohesive force;10 taken together with the present findings, it appears that interfibrillar cohesion is the primary mechanism underlying CCL.

Figs 3A to H: FTIR imaging of total protein, collagen triple helix structures and aggregated protein in untreated cornea tissue sections and cornea tissue sections crosslinked with UV light and riboflavin treatment: (A to D) Not treated; (E to H) UV light and riboflavin treated; (A and E) HandE histology; (B and F) FTIR imaging of total protein, integrated area under the amide I band from 1,700 to 1,600 cm−1; (C and G) FTIR imaging of collagen triple helix structures, second-derivative intensity as 1,666 cm−1; (D and H) FTIR imaging of aggregated proteins, second-derivative intensity as 1,625 cm−1
In addition, FTIR data suggest that while cross-links between collagen fibrils are being produced by UV treatment, which serves to strengthen the cornea, alterations in the collagen secondary structure are not occurring. This outcome is promising from a health safety perspective, as alterations in the protein secondary structure could potentially lead to a loss of function. This is consistent with previous reports as well.10,11
It is also worth noting that the FTIR techniques used in the present study are highly sensitive for the detection of aggregated and denatured proteins. Collagen cross-linking treatment has the potential to damage a small amount of the collagen protein. By itself, this small amount of damage would not affect corneal clarity, however, as other intact proteins come into contact with protein aggregates, they too may become aggregated or denatured, ultimately affecting corneal clarity. Work published by Xia et al. suggests that CCL can lead to a wound healing response,16 described as a complex process involving keratocyte apoptosis, proliferation, and migration.19–21 Keratocytes are transformed into myofibroblasts, which are intrinsically involved in stromal remodeling, thus serving as major determinants of corneal transparency following CCL.19–21 The present data do not reveal any evidence of aggregated proteins; however, this is a delayed phenomenon that may not have been detected in this protocol.
LIMITATIONS
The primary limitation of this investigation was the inability to detect changes in intermolecular cross-linking directly. The absence of intramolecular bond changes strongly suggests that intermolecular changes must occur. No gradient in the treatment effect was detected, based on the depth at which treated corneal tissue was sampled, thus we were unable to quantify the depth to which CCL treatment penetrates the cornea.
The X-ray scattering beamline may be able to confirm our hypothesis. X-ray scattering can reveal, at atomic resolution, the distribution of atoms in proteins. The cross-linking process may change the X-ray scattering properties of collagen, enabling us to observe where the cross-linking has occurred.
CONCLUSION
Previous studies hypothesize that both intermolecular and intramolecular linkages of collagen fibrils are responsible for increased corneal strength posttreatment. Our data from HandE staining demonstrate that UV treatment strengthens corneal collagen. Fourier-transform infrared spectroscopy data obtained suggest that intramolecular cross-linking does not play a major role in CCL and that it is more likely an increase in intermolecular linkages that accounts for the change in corneal strength. Furthermore, FTIR imaging demonstrates that UV treatment does not significantly affect the secondary structure of collagen, nor does it denature collagen or produce protein aggregates in the tissue.
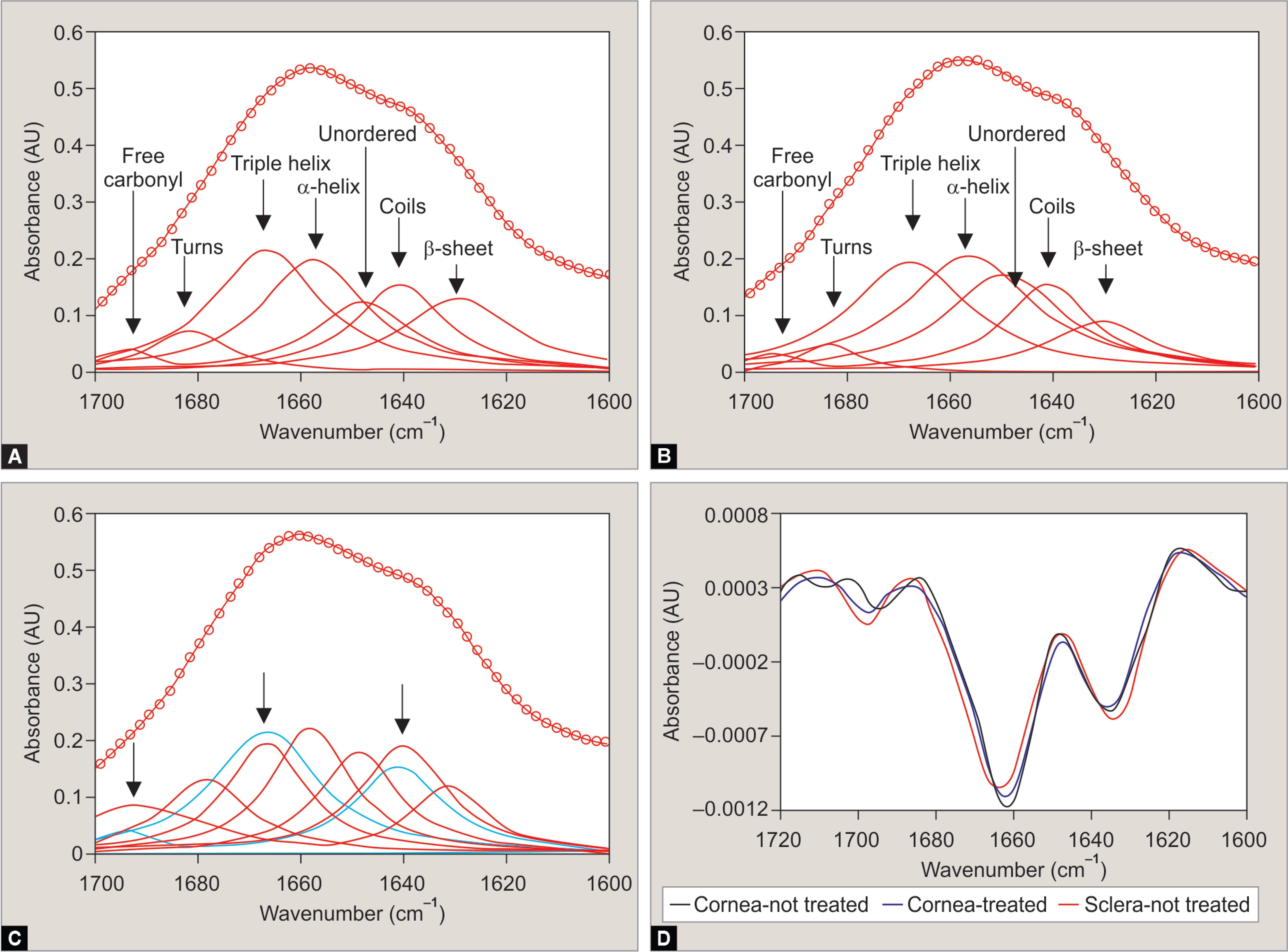
Figs 4A to D: FTIR spectra and curve fitting of the amide I band to reveal secondary protein structures in cornea tissue. Curve fitting of: (A) untreated cornea tissue; (B) UV riboflavin-treated cornea tissue; (C) Untreated sclera tissue; (D) Second-derivative analysis of the spectra presented in (A to C). The curve fitting of untreated and UV-treated cornea yield nearly identical fits. However, curve fitting of spectra collected from the sclera identified different secondary structures of the collagen protein, relative to the cornea. The differences were reflected in the second-derivative spectra
CLINICAL SIGNIFICANCE
Findings of the present investigation highlight that an increase intermolecular linkages between collagen fibrils likely accounts for the increase in corneal strength seen following CCL. We hope our work will guide future investigations in optimizing techniques of corneal CCL to maximize patient outcomes.
REFERENCES
1. Gordon-Shaag A, Millodot M, Shneor E, et al. The genetic and environmental factors for keratoconus. Biomed Res Int 2015;2015: 795738. DOI: 10.1155/2015/795738.
2. Mas Tur V, MacGregor C, Jayaswal R, et al. A review of keratoconus: diagnosis, pathophysiology, and genetics. Surv Ophthalmol 2017;62(6):770–783. DOI: 10.1016/j.survophthal.2017.06.009.
3. Chang S-H, Mohammadvali A, Chen K-J, et al. The relationship between mechanical properties, ultrastructural changes, and intrafibrillar bond formation in corneal UVA/riboflavin cross-linking treatment for keratoconus. J Refract Surg 2018;34(4):264–272. DOI: 10.3928/1081597X-20180220-01.
4. Andreassen TT, Simonsen AH, Oxlund H. Biomechanical properties of keratoconus and normal corneas. Exp Eye Res 1980;31(4):435–441. DOI: 10.1016/s0014-4835(80)80027-3.
5. Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol 2006;17(4):356–360. DOI: 10.1097/01.icu.0000233954.86723.25.
6. Spörl E, Huhle M, Kasper M, et al. Increased rigidity of the cornea caused by intrastromal cross-linking. Ophthalmologe 1997;94(12):902–906. DOI: 10.1007/s003470050219.
7. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 2003;135(5):620–627. DOI: 10.1016/s0002-9394(02)02220-1.
8. Medeiros CS, Giacomin NT, Bueno RL, et al. Accelerated corneal collagen crosslinking: technique, efficacy, safety, and applications. J Cataract Refract Surg 2016;42(12):1826–1835. DOI: 10.1016/j.jcrs.2016.11.028.
9. Kymionis GD, Kontadakis GA, Hashemi KK. Accelerated versus conventional corneal crosslinking for refractive instability: an update. Curr Opin Ophthalmol 2017;28(4):343–347. DOI: 10.1097/ICU.0000000000000375.
10. Wollensak G, Sporl E, Mazzotta C, et al. Interlamellar cohesion after corneal crosslinking using riboflavin and ultraviolet A light. Br J Ophthalmol 2011;95(6):876–880. DOI: 10.1136/bjo.2010.190843.
11. Raiskup F, Spoerl E. Corneal Crosslinking with Riboflavin and Ultraviolet A. I. Principles. Ocul Surf 2013;11(2):65–74. DOI: 10.1016/j.jtos.2013.01.002.
12. Gaster RN, Caiado Canedo AL, Rabinowitz YS. Corneal collagen cross-linking for keratoconus and post-LASIK ectasia. Int Ophthalmol Clin 2013;53(1):79–90. DOI: 10.1097/IIO.0b013e3182773ab4.
13. Kato Y, Uchida K, Kawakishi S. Aggregation of collagen exposed to UVA in the presence of riboflavin: a plausible role of tyrosine modification. Photochem Photobiol 1994;59(3):343–349. DOI: 10.1111/j.1751-1097.1994.tb05045.x.
14. Marcovich AL, Brandis A, Daphna O, et al. Stiffening of rabbit corneas by the bacteriochlorophyll derivative WST11 using near infrared light. Invest Opthalmol Vis Sci 2012;53(10):6378–6388. DOI: 10.1167/iovs.12-9913.
15. Balasubramanian D, Kanwar R. Molecular pathology of dityrosine cross-links in proteins: structural and functional analysis of four proteins. In: ed. V, Vallyathan X, Shi V, Castranova ed. Oxygen/Nitrogen Radicals: Cell Injury and Disease. Boston, MA: Springer US; 2002. pp. 27–38. DOI: 10.1007/978-1-4615-1087-1_4.
16. Xia Y, Liu B, Fan Z, et al. Corneal collagen fibril changes after ultraviolet a/riboflavin corneal crosslinking. Cornea 2014;33(1):56–59. DOI: 10.1097/ICO.0000000000000017.
17. Wollensak G, Wilsch M, Spoerl E, et al. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea 2004;23(5):503–507. DOI: 10.1097/01.ico.0000105827.85025.7f.
18. Choi M, Kim J, Kim EK, et al. Comparison of the conventional Dresden protocol and accelerated protocol with higher ultraviolet intensity in corneal collagen cross-linking for keratoconus. Cornea 2017;36(5):523–529. DOI: 10.1097/ICO.0000000000001165.
19. Netto MV, Mohan RR, Ambrósio R, et al. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea 2005;24(5):509–522. DOI: 10.1097/01.ico.0000151544.23360.17.
20. Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg 2009;35(8):1358–1362. DOI: 10.1016/j.jcrs.2009.03.035.
21. Wilson SE, Kim WJ. Keratocyte apoptosis: implications on corneal wound healing, tissue organization, and disease. Invest Ophthalmol Vis Sci 1998;39(2):220–226.
________________________
© The Author(s). 2019 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.